How to Find Vapor Pressure From Molality
Mass solvent 250. The molality formula is given below.

Mole Fraction Of Solute The Vapour Pressure Of Pure Benzene At 25 C Is 639 7 Mm Of Hg And The Vapour Pressure Of A Soluti Of A Solute In C H At
Calculate the change in boiling or freezing temperature using one the following formulas.

. Enter the value of Pressure P Reset calculator for new calculation. Add this increase to the boiling point of pure water to find the boiling point of the solution. Next use the molality vant Hoff factor and boiling point elevation constant to solve for the increase in boiling point.
Find the molarity of 214 m HF. Enter a temperature or a dewpoint or both. Moles solute 182 g 1622 gmol 0112207 mol.
If the vapor pressure of the solution is 2351 torr into how many ions does the electrolyte dissociate. The molality of a saturated solution of AlNO 3 3 9H 2 Os at T 29815 K is 3161 molkg 1 in which the mean ionic activity coefficient γ is 116 and the vapor pressure is 19158 kPa. Raoults law is a chemical law that states that the vapor pressure of a solution is dependent on the mole fraction of a solute added to the solution.
P solution Χ solvent P 0 solvent. P is the Pressure m is the Molality M is the Molar Mass of Solvent Ps is the Pressure of Solution frac P - P_s P_s i times frac n N Enter the value of known variable to calculate unknown variable. This is equal to 0862 kg H 2 O Molality moles H 2 SO 4 1 kg H 2 O 375 moles 0862 kg 435 m H 2 SO 4.
G solution - 368 g solute 862 g H 2 O solvent. This is a very important step and the amount of solution is not given but you need to have a specific quantity to do the. The mechanism behind boiling point elevation can be understood by examining another colligative property vapor pressure.
Molality is mainly used when working with a range of. ΔTf Kf m or ΔTb Kb m. How do you find boiling point from molarity.
1 182 means this. The SI unit for molality is molkg and unlike molarity which depends on the volume of the solution molality depends only on the mass of the solvent. Convert grams of glucose to.
As more solute is dissolved in the solvent the vapor pressure of the solvent decreases and the change in the vapor pressure of the solvent increases. Mb in of HG mm HG. Determine the van t Hoff factor.
At this temperature pure water has a vapor pressure of 3167 kPa. Calculate the molality of the NaCl. A Calculate the osmotic coefficient of a saturated solution of AlNO 3 3.
The mole fraction simply refers to ratio between solvent moles and the total number of moles in the solution. Assume you have 1 kg of solvent water. Vapor pressure of the solution mole fraction of solvent vapor pressure of the pure solvent This is known as Raoults Law.
When the vapor pressure of a liquid is increased to the atmospheric pressure by heating it boils. Mass solute 2860 g C 6 H 12 O 6. 182 g solute in 100 g of solution.
Must be in Kelvins so 20 2731529315 K molar mass of. G 0250 kg. Molar mass C 6 H 12 O 6 18018 gmol.
Molality m of NaCl moles of NaClkg water From the periodic table find the atomic masses of the. If you want the saturated vapor pressure enter the air temperature. P solution χsolvent P 0 solvent χsolute.
078 m This is the question and so far Im so confused. This chemistry video tutorial provides a basic introduction into Raoults law which says that the vapor pressure of a solution is the product of the mole fra. Heres the above molality calculation shown more visually.
Mole fraction ethanol --- 0883 0883 0334 0726. Determine the molality of a solution prepared by dissolving 2860 g of glucose C 6 H 12 O 6 into 250. Moles naphthalene --- 4271 g 128 gmol 0334 mol.
By Tim Brice and Todd Hall. Conversion from Molality to Molarity Problem. This aqueous solution has a density of 1101 gmL.
Molality Calculations To calculate molality we need moles of solute which we have and kg solvent. 1701torr 1760atm02238 atm R is a constant at 08205745 Temperature. Molality or molal concentration is a measure of the concentration of a solute in a solution in terms of amount of substance in a specified amount of mass of the solvent.
Left mathsf m right dfrac mathsf molesofsolute mathsf kilogramsofsolution. HPA kPA lbs per square in. Raoults Law is expressed by the formula.
Calculate the molality of the solution. This demonstration can also be usde to explain Roults law - colligative property. When a non-volatile solute is added to a pure liquid the entropy of the system is increased by mixing and the rate of evaporization is decreased leading to the.
The vapor pressure of water at this temperature is 2602 torr. Vapor pressure which can be found using Raoults law is a measure of how. Moles ethanol --- 4065 g 4602 gmol 0883 mol.
If however youre dealing with a solution that contains a volatile solute the vapor pressure of that solution is. To calculate the molality we need to find moles of solute per kilogram of solution. List the known quantities and plan the problem.
First of all I know that moles npressurevolumeRtemperature pressure. Therefore 818 g of water.

Chem 201 Calculating Vapor Pressure Of A Solution With Two Volatile Components Youtube

203 Solutions 2 Molality Vapour Pressure Youtube
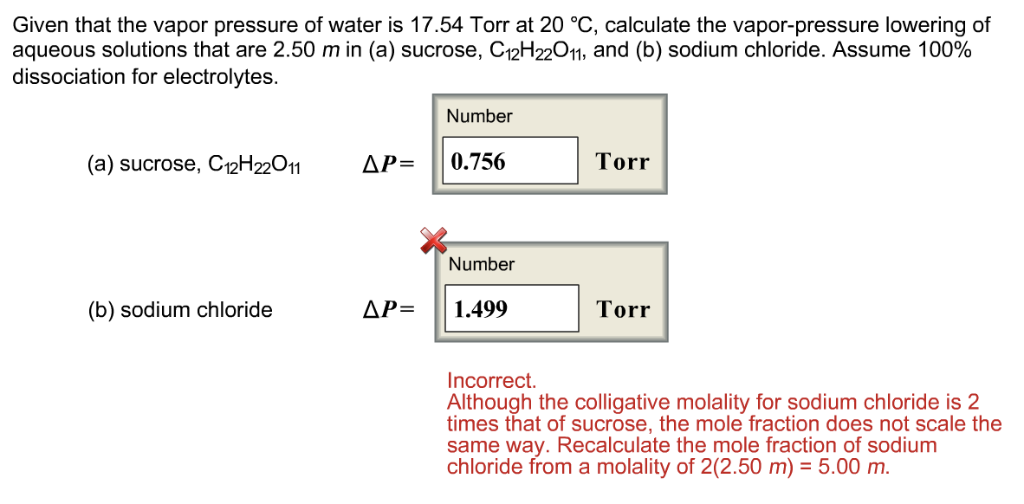
Solved Given That The Vapor Pressure Of Water Is 17 54 Torr Chegg Com

Vapor Pressures Of Solutions Ppt Download
Find The Molality Of A Solution Containing A Non Volatile Solute If The Vapour Pressure Is 2 Below The Vapour Pressure Of Pure Water Sarthaks Econnect Largest Online Education Community

The Relative Lowering Of The Vapour Pressure Of An Aqueous Solution Containing A Non Volatile So Youtube

Mullis1 2 Concentration Measurements Molarity M Moles Of Solute Volume Of Solution In L Molality M Moles Of Solute Mass Of Solvent In Kg Mole Ppt Download

The Vapour Pressure Of An Aqueous Solution Of Glucose Is 750 Mm Of Hg At 373 K Calculate Youtube

Raoult S Law How To Calculate The Vapor Pressure Of A Solution Youtube

Raoults Law And Vapor Pressure Chemistry Tutorial Youtube

Calculate The Vapour Pressure Of An Aqueous Solution Of 1 0 Molal Glucose Solution At 100 C Youtube
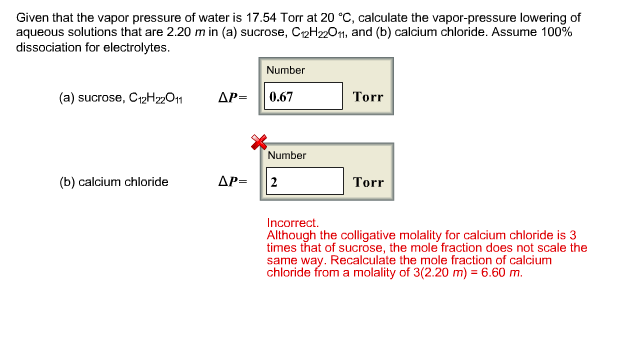
Solved Given That The Vapor Pressure Of Water Is 17 54 Torr Chegg Com

Solved A 13 0 Aqueous Solution Of Kzcoz By Mass Has A Density Of 1 09 G Cm Calculate The Molality Of The Solution 12 Pts Calculate The Vapor Pressure Of Water In Mmhg At 7 00

The Vapour Pressure Of Pure Benzene At 25 C Is 639 7 Mm Of Hg And Vapour Press Youtube

Vapor Pressure Of Cacl2 At 3 89 Molality At Different Temperature Download Scientific Diagram

Chem 201 Calculating Vapor Pressure Of A Non Electrolyte Solution Using Raoult S Law Youtube
Comments
Post a Comment